VRML MODELS OF LITHIUM INSERTION IN 3D-NETWORKS OF TRANSITION METAL MOLYBDATES
N.G. Sudorgin
7, Zorge, Chemistry
department of Rostov State University, Rostov-on-Don,
Russia,
sng@rnd.runnet.ru
3D-networks, aluminum, chemistry of solid, chromium, crystalline lattice, crystallography, electrochemical, insertion, ion, ion transport, iron, lithium, molybdates, monoclinic, Netscape, neutron, orthorhombic, phases, power diffraction, structure, transition metal, virtual reality, visualization, VRML, X-ray, zirconium.
Development of computer technologies has brought to a creation of the new forms of presentation scientific information. Amongst them particular interest present methods virtual reality. Their using allows find a complex decision of number of problems, in accordance with processing and publication of results of scientific studies in the field of natural sciences and, in particular, in crystallography and chemistry of solid.
In this paper are present results a structure
modeling of chromium, aluminum, iron and zirconium (low
temperature modification) molybdates. As objects for
visualizations are choose materials, in detail studied by several
physicists-chemical and electrochemical methods [1,2,3,4,5].
Original phases of tree-valence metal is devoted to Sc2(WO4)3
structure type with monoclinic cell of P21/c specious
group and original LT-Zr(MoO4)2 has owner
structure type with monoclinic cell of C2/c specious group (table
1). For all of them it is distinctive an ability of effectively
to insert of lithium ions in its crystalline lattices. Changes of
cell parameters by the insertion for all except ion molybdate are
imperceptible. Such behavior may be explained by the existence in
crystal structures of these materials of specious cavities
bounded between its sufficiently passages. The necessary of
testing this hypothesis does given group of materials highly
attractive for the VRML-modeling. The advisability of use not
only real, as well as virtual models, is bound with big sizes and
complex structures of elementary cells given materials. For the
best understanding the mechanisms of moving the lithium ions in
structures is advisable to visual possible ways of the lithium
ion transport within the whole channel system by step by step
scanning.
Table 1. X-ray power diffraction data for LiXM2(MoO4)3 (*) and LT-LiXM(MoO4)2 (**).
| M | X | Cell | a,Å | b,Å | c,Å | âo |
| Al * | 0 | Monoclinic P21/c | 15,40(6) | 8,99(3) | 17,93(5) | 125,5(4) |
| Al * | 3 | Monoclinic P21/c | 15,40(6) | 8,99(3) | 17,94(6) | 125,5(5) |
| Fe * | 0 | Monoclinic P21/c | 18,27(3) | 9,23(1) | 15,72(2) | 125,5(2) |
| Fe * | 2 | Orthorhombic Pbcn | 12,86(11) | - | 9,43(8) | 9,37(8) |
| Cr * | 0 | Monoclinic P21/c | 18,14(3) | 9,15(1) | 15,58(2) | 125,4(2) |
| Cr * | 2,3 | Monoclinic P21/c | 18,12(7) | 9,16(3) | 15,62(6) | 125,4(5) |
| Zr ** | 0 | Monoclinic C2/c | 11,43(4) | 7,94(2) | 7,60(2) | 122,1(4) |
| Zr ** | 2 | Monoclinic C2/c | 11,52(2) | 7,93(1) | 7,62(1) | 122,3(2) |
The modeling was realized in the format VRML ver.1.0. Data about atomic coordinates were take from literary sources [3,4,5]. The generation of atoms was run for the program, written by A.I. Lujetsky on Pascal. VRLM-files were produce by means of the program-converter, written by author on Basic. The visualization of structures was realized by means of Netscape ver. 3.0 and above. A scene from different standpoints was copy from the screen in the file and at need was printed.
The visualization of structures a molybdates
trivalent metals shows on a presence of large cavities, bound
between itself channels at all M2(MoO4)3.
The insertion of lithium doesn't change topology of all
3D-networks. Such behavior makes possible to use atom coordinates
data of original phases for the modeling of phases after
insertion. Although neutron power diffraction data by lithium ion
coordinates are only for iron molybdate it is possible to finger
the preference position by using of the visualization and for
other substances. Array of such positions is akin to its which
observe after the insertion in iron molybdate. The lithium ions
orderly dispose in the most spacious cavities. Possible
differences between substance may be clear by influence electron
structures of according transfer metal ions. In the structure of
low temperature zirconium molybdate cavities far less than other
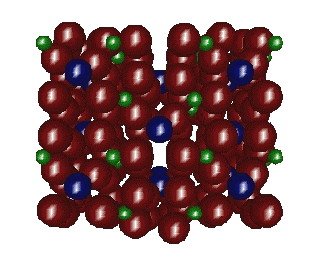
structures, but channels are present too (Fig.1.). This result is
in a good agreement with data, received by study of reactions of
electrochemical insertion lithium [2].

Fig. 1. Projection of the structure of LT-Zr(MoO4)2 along [001] direction.
Received results show that preliminary
suggestions on presence of channels in crystalline structures of
studied substances are correct. This confirms, offered earlier
mechanisms of chemical conversions [2]. In the same time, absence
of efficient ways of quantitative interpreting the results to
visualizations limits the possibilities of method and requires
new studies.