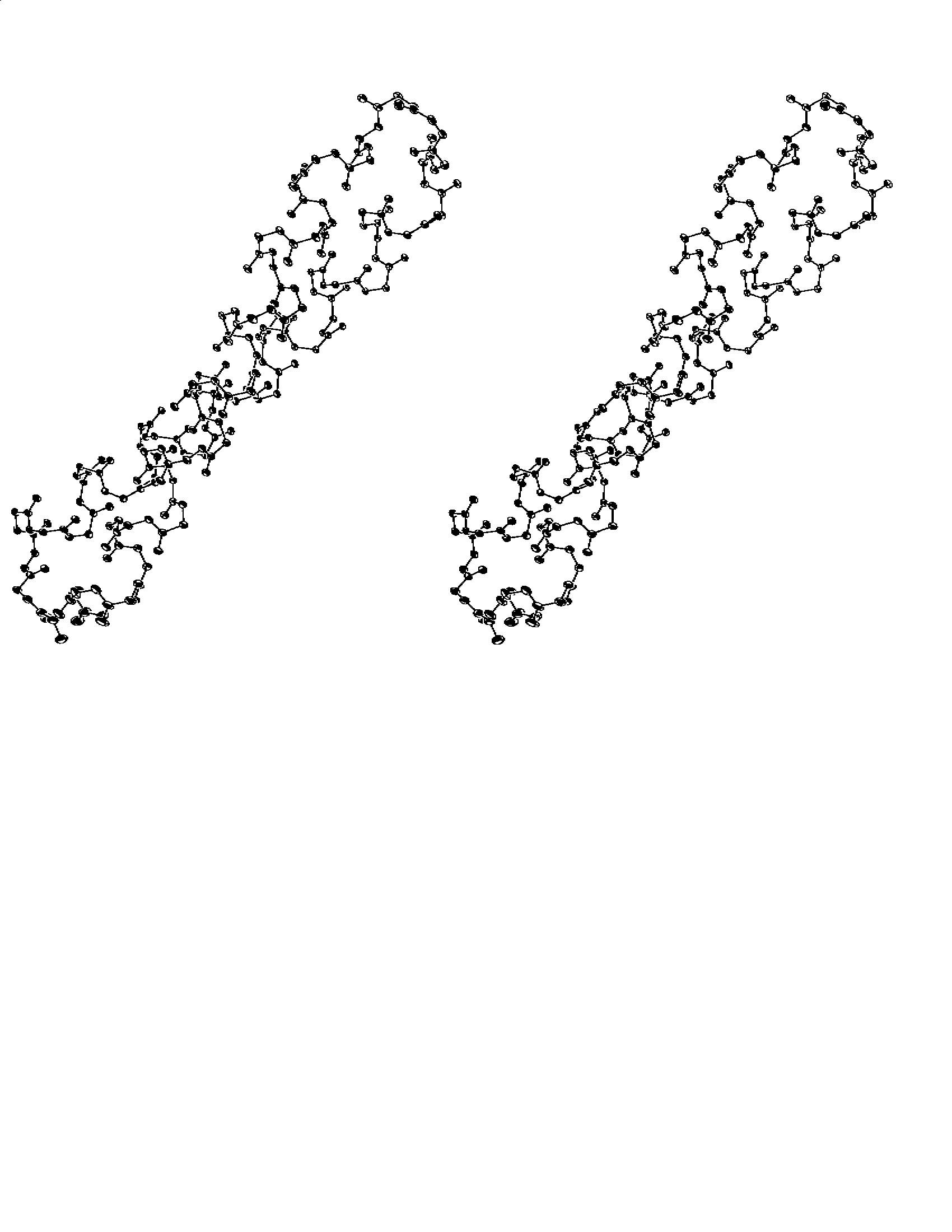
STRUCTURAL PARAMETERS FOR PROTEINS DERIVED FROM THE ATOMIC RESOLUTION (1.09A) STRUCTURE OF DESIGNED VARIANT OF THE ColE1 ROP PROTEIN
M. Vlassi1, M. Kokkinidis2,3
1 National Centre for
Scientific Research "Demokritos", 15310 Ag. Paraskevi
Attikis, Athens, Greece.
2 University of Crete,
Department of Biology, P.O. Box 1470, GR-71110 Heraklion, Crete,
Greece.
3 Institute of Molecular
Biology and Biotechnology, P.O. Box 1527, GR-71110 Heraklion,
Crete, Greece.
Keywords:
atomic resolution, unrestrained blocked-full matrix refinement,
anisotropic thermal parameters.
The crystal structure of a designed variant of the ColE1 Repressor of Primer (ROP) protein [1-2], which follows the 4-á-helical bundle motif, has been refined with SHELXL-93 [3] to a resolution of 1.09A. The final model with 510 non-hydrogen protein atoms, 576 hydrogens in calculated positions and 114 water molecules converged to a standard R factor of 10% using unrestrained blocked-full matrix refinement. For all non-hydrogen atoms 6-parameter anisotropic thermal parameters have been refined.
Atomic vibrations are clearly anisotopic with the thermal ellipsoids showing a directional preference of atomic fluctuations (Fig. 1) with the major vibrational axes being almost perpendicular to the bundle axis. The directionality of the the anisotropy is dictated from the crystal packing. Analysis with the TLS Method [4] showed relatively good agreement between the individual atomic motion and a rigid-body motion.
The unrestrained blocked-full matrix least squares refinement yielded reliable estimates of the standard deviations of the refined parameters. Comparison of these parameters with the stereochemical restraints used in various protein refinement programs showed statistically significant differences. Therefore, new values for stereochemical parameters derived from atomic-resolution protein structures should be adopted as restraints, a suggestion also made in earlier [5-6]. Alternatively, weighting schemes based on the expected standard deviations of the stereochemical parameters could replace the uniform weighting used so far in refinement.
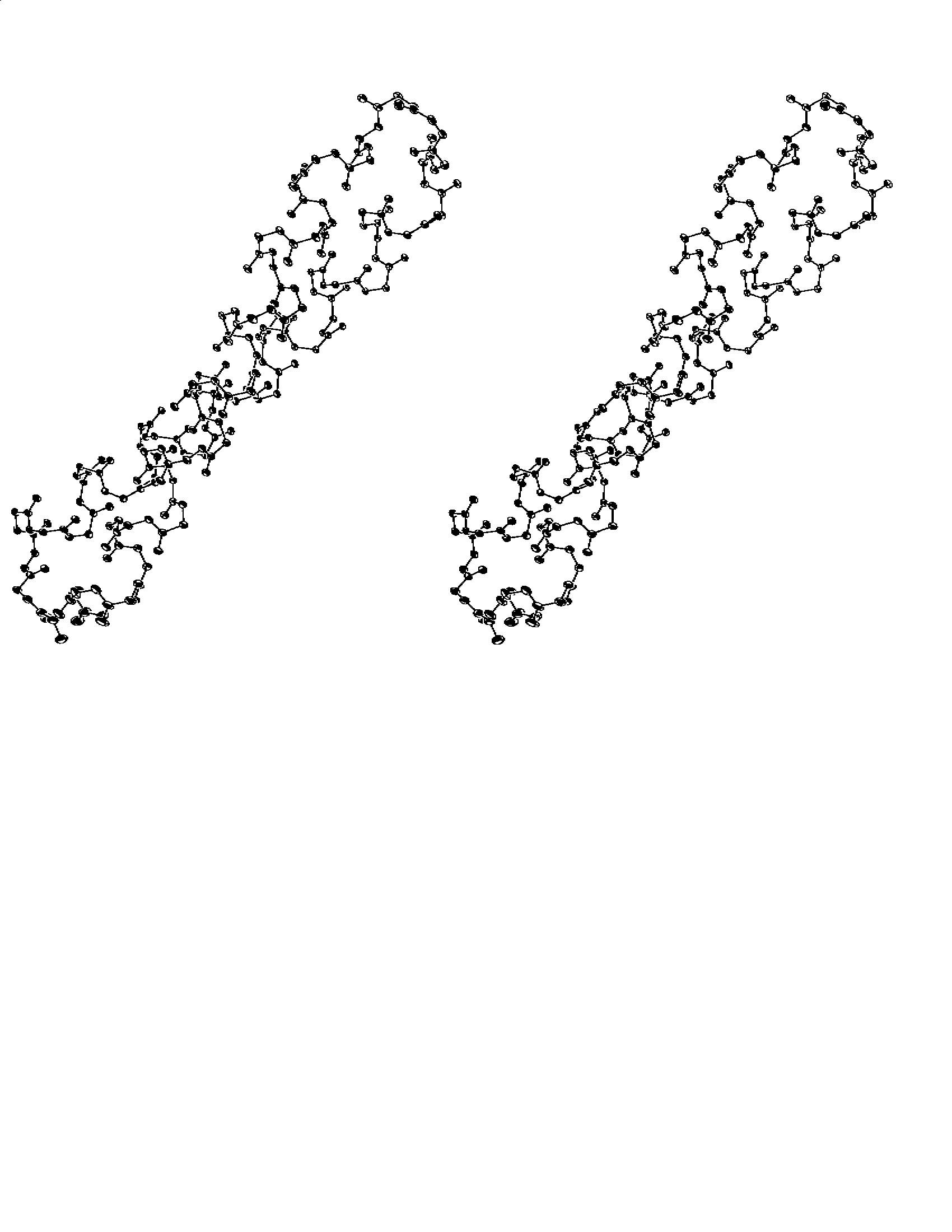
Fig. 1. Thermal
ellipsoids (calculated at 99% probability level) for the backbone
atoms of the <2aa> mutant of ROP. The more pronounced
character of atomic motion along one direction is due to crystal
packing effects.
1. D.W. Banner, M.
Kokkinidis, D. Tsernoglou: J. Mol. Biol., 196 (1987),
657-675.
2. M. Vlassi, C. Steif, P. Weber, D. Tsernoglou, K. Wilson, H-J.
Hinz, M. Kokkinidis: Nat. Structural Biology 1,
no 10, (1994), 706-716.
3. G.M. Sheldrick: SHELXL-93, Program for crystal structure
refinement, University of Gottingen, Germany, (1993).
4. V. Schomaker, K.N. Trueblood: Acta Cryst. B24,
(1968), 63-77.
5. R.A. Engh, R. Huber: Acta Cryst. A47,
(1991), 392-400.
6. R.A Laskowski, D.S. Moss, J.M. Thornton: J. Mol. Biol.
231, (1993), 1049-1067.