THE USE OF THE ELECTRONIC LOCALISATION FUNCTION AND PARTIAL ELECTRONIC DENSITY FOR INVESTIGATING THE ELECTRONIC STRUCTURE OF THE ZINTL ANIONS IN BA3GE4
Fabio Zürcher, Reinhard Nesper
Laboratorium für Anorganische Chemie; ETH
Zürich; Universitätstrasse 6; CH-8092 Zürich; Switzerland
Keywords: Zintl Phases, Electronic Localisation
Function, Partial Electronic Density
The Zintl phases are intersting materials because
of the almost infinite structural variety of their Zintl anions.
The Zintl phase Ba3Ge4 [1] crystallises in
a new structure type in the orthorhombic space group Cmmm.
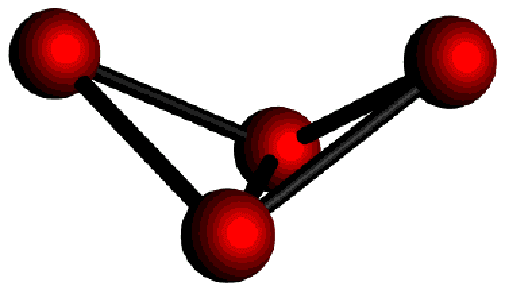
It contains isolated ![]() butterfly anions (Fig.1) and butterfly anions that are
polymerised to infinite chains
butterfly anions (Fig.1) and butterfly anions that are
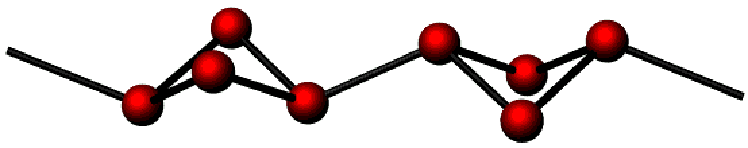
polymerised to infinite chains ![]() (Fig.2). In a concerted way an intermolecular bond
is formed while the central intramolecular bond is broken. The
structure of Ba3Ge4 is one of the few
example of a polymerisation in the solid state. One of the most
interesting characteristic of this structure is the presence of a
reversible polymerisation in a single crystal, i.e. the polymer
and the isolated monomers cohexist in the same crystal. It is
also one of the few example of bond length isomerisation in the
solid state. It is interesting to note that the intermolecular
bond that connects the butterfly anions has a very long bond
distances (287 pm).
(Fig.2). In a concerted way an intermolecular bond
is formed while the central intramolecular bond is broken. The
structure of Ba3Ge4 is one of the few
example of a polymerisation in the solid state. One of the most
interesting characteristic of this structure is the presence of a
reversible polymerisation in a single crystal, i.e. the polymer
and the isolated monomers cohexist in the same crystal. It is
also one of the few example of bond length isomerisation in the
solid state. It is interesting to note that the intermolecular
bond that connects the butterfly anions has a very long bond
distances (287 pm).
 |
 |
| Fig.1: isolated |
Fig.2: polymerised |
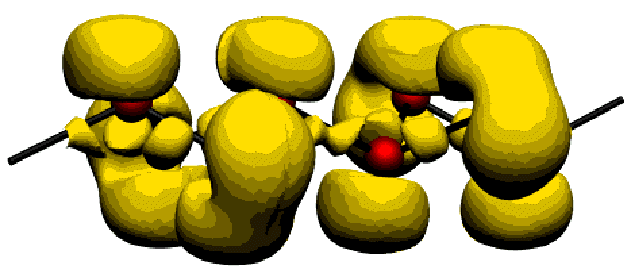
The electron localisation function (ELF) has already demonstrated to be an extraordinary powerful tool for investigating bonding structures for many different chemical systems by giving an easily and directly understandable picture of the chemical bond [2,3].
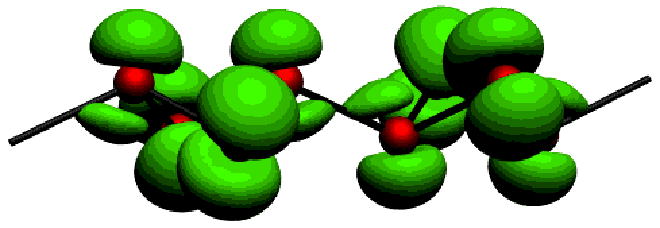
The partial electron density (PED) is an electronic density calculated in a selected energy window and it can be drawn as a 3-dimensional isosurface. It is suitable for giving a picture of the occupied orbitals in the chosen energy window.
The ELF is used to investigate the bonding structure of the Zintl anions contained in Ba3Ge4 (Fig.3). For a better undestanding of the bonding structure near the Fermi level, the use of the PED is shown here (Fig.4).
Of particolary interest is the understanding of
the intramolecular and intermolecular bond of the isolated and
polymerised buttefly anions, respectively.
 |
 |
| Fig.3: ELF of the |
Fig.4: PED of the |