EXPERIMENTAL CHARGE DENSITY STUDY OF THE METAL-METAL BONDING IN BINUCLEAR METAL CARBONYLS
Bianchi R.1, Gervasio G.2 and Marabello D.2
1 Centro CNR per lo Studio delle
Relazioni tra Struttura e Reattivita Chimica, Milano, Italy.
2 Dipartimento di Chimica I.F.M.
dell'Universita, Torino, Italy.
The experimental deformation charge density of some binuclear metal carbonyls, [CpFe(CO)2]2 , (m-CH2)[CpMn(CO)2]2 and Mn2(CO)10 , was determined by X-ray diffraction some years ago [1-3]. The theoretical deformation density for other similar complexes, Fe2(CO)9 and Co2(CO)8 , and for Mn2(CO)10 were reported elsewhere [4-5]. In all the three complexes examined by X-ray diffraction, the deformation maps showed no evidence of significant density accumulation in the metal-metal bonding region. This result was justified by the presence of bridging groups (CH2 or CO), which join the two metallic fragments to form the entire molecule. In the case of Mn2(CO)10 [6], which has only terminal carbonyl groups, a theoretical charge density study, based on the Bader's Quantum Theory of Atoms in Molecules (QTAM), provided the existence of the Mn-Mn bond.
The experimental charge density of two binuclear metal carbonyls, one containing bridging groups [Co2(CO)6(m-CO)(m-C4O2H2) ] (1) and the second having only terminal ligands [Mn2(CO)10] (2) was determined by accurate X-ray data, measured at low temperature and interpreted by the aspherical-atom formalism developed by Stewart [7]. Further, the results of the Bader analysis on the experimental charge density are reported, in order to better characterize the metal-metal bonding.
The topology of the charge density, r(r),
and its Laplacian,![]() 2(r),
has been fully described by Bader [8] in the QTAM: it has been
shown that a necessary and sufficient condition for two atoms to
be involved in a bonding interaction is the existence in r(r), of a line, linking the nuclei,
along which the charge density is at a maximum with respect to
any lateral displacement. Such line is called a bond path, and
the interatomic critical point rc ( where
2(r),
has been fully described by Bader [8] in the QTAM: it has been
shown that a necessary and sufficient condition for two atoms to
be involved in a bonding interaction is the existence in r(r), of a line, linking the nuclei,
along which the charge density is at a maximum with respect to
any lateral displacement. Such line is called a bond path, and
the interatomic critical point rc ( where ![]() (rc) =
0) occurring on it is a bond critical point (bcp). At the bcp,
there are two negative curvatures (l1
and l2) that determine the
contraction of towards rc in the directions
perpendicular to the bond path, and one positive curvature, l3, parallel to the interaction
line. The values of (rc) and of
(rc) =
0) occurring on it is a bond critical point (bcp). At the bcp,
there are two negative curvatures (l1
and l2) that determine the
contraction of towards rc in the directions
perpendicular to the bond path, and one positive curvature, l3, parallel to the interaction
line. The values of (rc) and of
![]() 2r(rc) =
l1 + l2 + l3 characterize the atomic
interaction.
2r(rc) =
l1 + l2 + l3 characterize the atomic
interaction.
a) |
b) |
 |
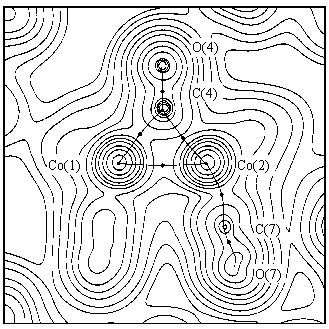 |
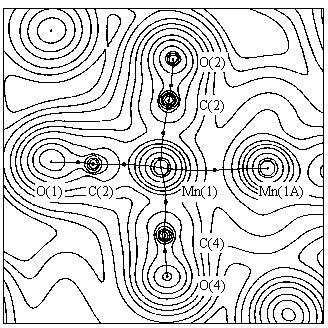
The figures a) and b) show the experimental charge density for the complexes (1) and (2), respectively, and the corresponding bond paths in a plane containing the two metal atoms.
The positive values of the Laplacian of r(r)
at the metal-metal bcp's (3.0(1) and 1.2(3) eA-5 for (1)
and (2), respectively) indicate closed shell interactions
[9] for the Co-Co and Mn-Mn bonds. The trend of ![]() r2(r)
in these regions shows that the density is contracted towards
each metal nucleus. This behaviour is similar to the ionic and
Van der Waals interactions and clearly indicates a totally
different nature with respect to a typical covalent interaction.
r2(r)
in these regions shows that the density is contracted towards
each metal nucleus. This behaviour is similar to the ionic and
Van der Waals interactions and clearly indicates a totally
different nature with respect to a typical covalent interaction.
A characterization of the metal-ligand bonds is also reported.