EXPERIMENTAL
AND THEORETICAL INVESTIGATION OF ELECTRON DENSITY DISTRIBUTION IN
MEISENHEIMER COMPLEX DERIVATIVE OF 5,7-DINITROQUINOLINE.
Oleg Ya. Borbulevych, Oleg V. Shishkin, Michael Yu. Antipin, Konstantin.A. Lysenko and Viktor N. Knyazev
A.N.Nesmeyanov
Institute of Organoelement Compounds, Russian Academy of
Sciences, 28 Vavilov St., Moscow 117813, Russia
Institute of Single Crystals, National Academy
of Sciences of the Ukraine, 60 Lenina ave., Khar'kov 310001,
Ukraine
K.A. Timiryazev
Agriculture Academy, Department, of organic chemistry, 49
Timiryazevskaya st, Moscow 127550, Russia.
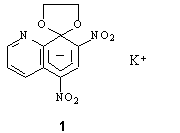
Molecular, crystal structure and an electron density distribution in the crystal of Meisenheimer complex Potassium (1,3 dioxolane)-2-spiro-8'-(5',7' dinitroquinolinide) 1 were studied using precision X-ray diffraction method at 153K.

Bader's topological theory was applied to theoretical study of the electron density distribution in anion 1 and in various complexes anion 1- K+ (with different coordination of the cation to oxygen atoms to take place in the crystal phase). For this purpose, ab initio calculations of the wave functions at the HF/6-311G* level of theory was performed.
A six-membered ring of 1 containing spiroatom has the structure close to 1,4-cyclohexadiene. The nitro groups are not equivalent. In spite of existence of alternation of the bond length in the ring mentioned, there is no essential difference of the heights of the deformation electron density (DED) maxima in the ring bonds. But DED maps confirm non-equivalent character of the nitro groups. Peak of DED at the C-N bond of the nitro group attached to the C-5 carbon is almost twice higher then that of the nitro group attached to the C-7 carbon. Experimental and theoretical results have shown that the magnitude of ED at the critical point of the C(5)-N bond is little more then that of the C(7)-N bond as well. However, according to experimental data, ellipticity of the C(5)-N bond is less than that of the C(7)-N bond. This result contradicts our theoretical data for 1, where inverse ratio between ellipticities of these bonds was observed. But theoretical topological analysis of complexes anion 1 - K+ has revealed that ellipticity is very sensitive to the influence of a cation. Taking into account the effect of all potassium atoms connected to the molecule anion in the crystal excellent agreement between experiment and theoretical data concerning ellipticity of the mentioned C-N bond was obtained.